

Copyright©IBMI&RBMP
According to Clinical.gov, more than 700 new drugs against Covid-19 developed and registered by companies worldwide are undergoing Phase 2 and Phase 3 clinical trials. More than 500 clinical trials are underway around the globe with unprecedented competition. This article provides an overview of the current progress in drug development worldwide in three dimensions, including (1) FDA approvals, (2) oral dosage forms and (3) the R&D of new drugs in Taiwan’s bio-tech companies.
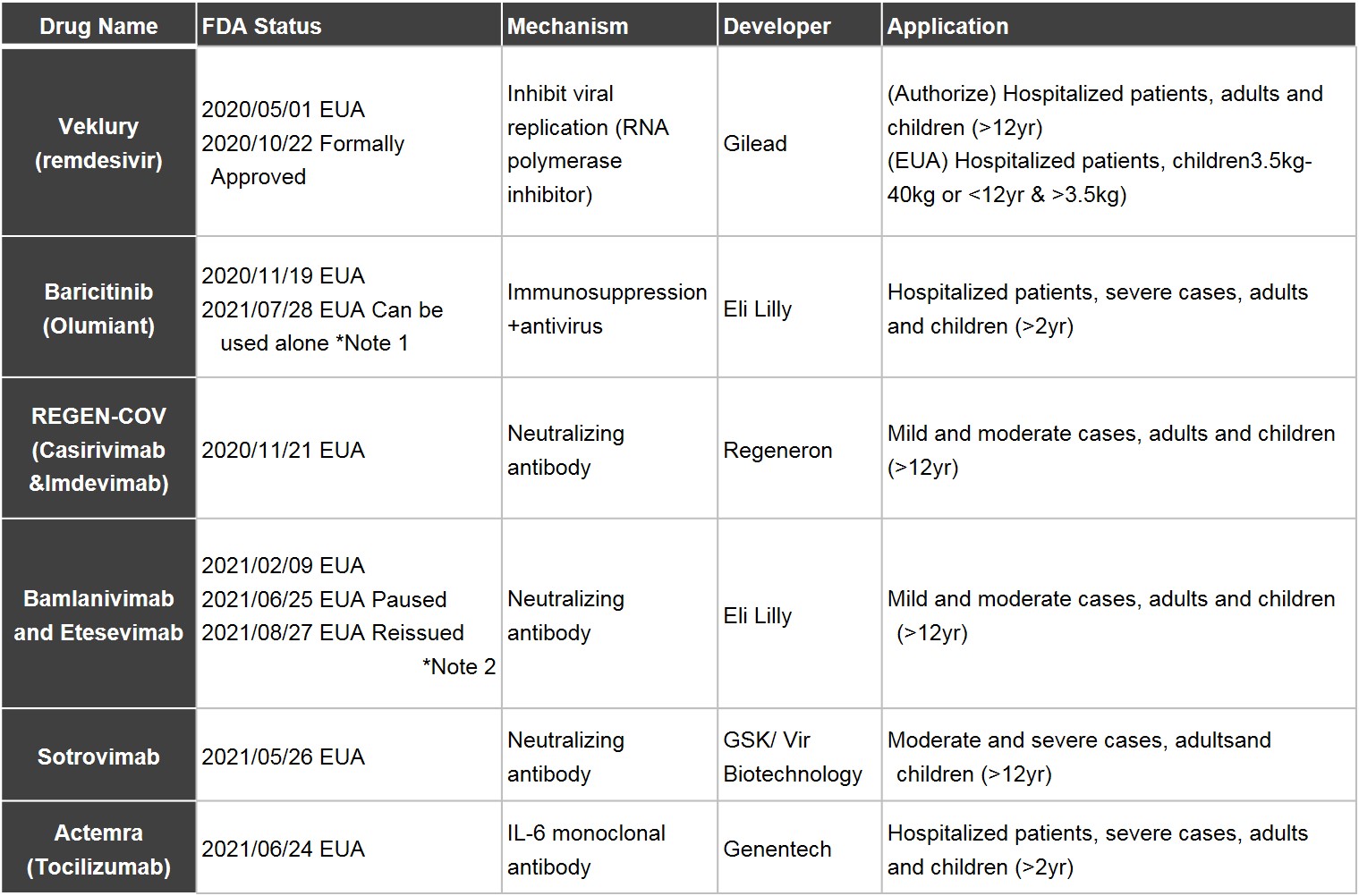
■ One durg with FDA approval, five with EUA, yet only one oral-administered
In May 2020, Gilead's Remdesivir became the first FDA-approved treatment of COVID-19 under EUA. Remdesivir, an RNA polymerase inhibitor, was originally developed against Ebola virus. During the epidemic, the Phase 3 clinical trials enrolled 4,891 patients with severe symptoms and 1,113 patients with moderate symptoms, and obtained approval for the drug’s use on hospitalized patients who are older than 12 or are adult patients in October the same year. As for children with lower weights or younger age, the use is still in compliance with EUA.
For the time being, most enterprises granted EUA are well-known international pharmaceuticals, including Eli Lilly, which received two EUAs (Baricitinib, Bamlanivimab and Etesevimab); GSK, which collaborated with Vir Biotechnology to develop neutralizing antibody Sotrovimab to inhibit viral entry into the cell; Regeneron, a big biopharmaceutical company which received EUA for REGN-COV Antibody Cocktail, the combination of two monoclonal antibodies Casirivimab and Imdevimab. The latest EUA was granted to Genentech, a subsidiary of Roche, which developed the subcutaneous injection of IL-6 monoclonal antibody. The mechanism is to prevent the Cytokine Storm from happening and the deterioration in severe cases through inhibition of IL-6.
Currently, among most of the FDA-approved drugs and those authorized under EUA are old drugs in clinical use, antiviral drugs under development, immunomodulators and antibody drugs. Mostly, the drugs are injectable, only Baricitinib is administered orally.
Table 1. COVID-19 Drugs Approved and Authorized under an EUA
Source: FDA, official websites in different companies, compiled by IBMI & RBMP
Note
1.Baricitinib, which obtained EUA for the first time in November 11, 2020, should be taken with remdesivir for the treatment of hospitlazed patients with severe symptoms; afterwards, FDA announced revisions in July 28, 2121, to permit the use of baricitinib monotherapy under EUA.
2.FDA granted EUA to the use of Bamlanivimab plus Etesevimab for the treatment of patients with mild and moderate symptoms; later, due to the inactivity of Bamlanivimab plus Etesevimab against variants, EUA was paused; revisions were made to permit its use in regions where variants infect less than 5% of the population in August 27, 2021.
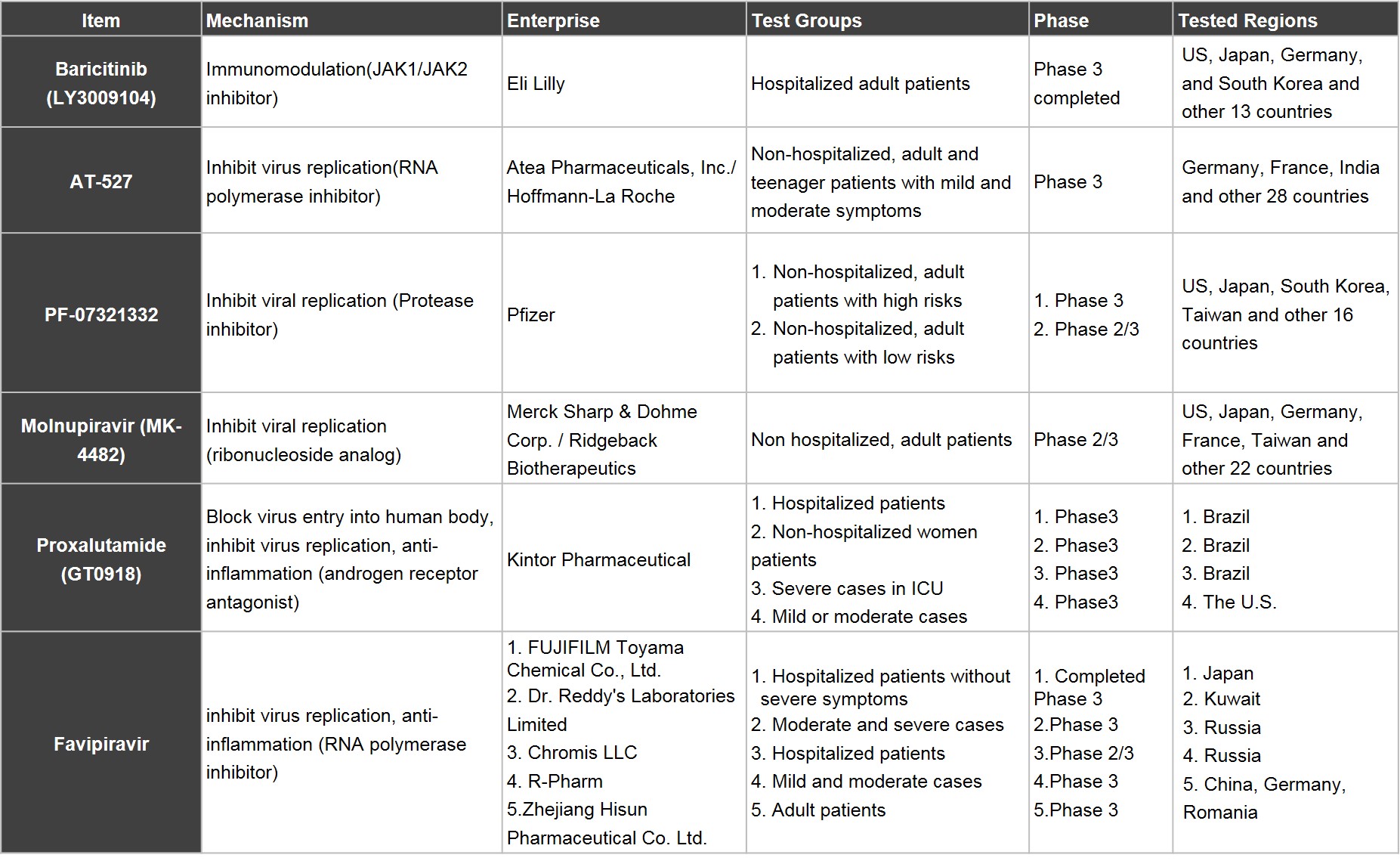
■ Front Runners in the R&D of New Oral Dosage Forms: Eli Lilly, Pfizer, Roche, and Merck Sharp & Dohme are in the last stage
For now, the number of Phase 3 clinical trials for Covid-19 launched by enterprises worldwide has exceeded 150, with most of them being injection dosage forms, only few are for oral dosage forms. The R&D of new Covid drugs focus on four dimensions, including (1) the treatment of hospitalized patients to prevent deterioration in severe cases, (2) the treatment of symptoms in severe cases, (3) the treatment of the infected to prevent from being hospitalized and severe cases, and (4) the disease prevention after exposure to virus.
Based on the progress of clinical trials, front-runners in the development of new oral drugs are still international well-renowned companies. Eli Lilly’s Baricitinib, which is immunomodulatory drug originally for the treatment of rheumatoid arthritis and it is the fastest one to received EUA. In this June, it completed Phase 3 clinical trial which recuited 1,585 participants and mainly focused on the prevention of severe case among hospitalised adults.
Pfizer, which made the successful bid to develop vaccines with BNT, and Roche, the successful developer of Tamiflu in the past, both have their shares in the development of new covid drugs. PF-07321332 developed by Pfizer, and AT-527 co-developed by Roche and Atea, are protease inhibitor and RNA polymerase inhibitor respectively, whose mechanisms are both to inhibit viral replication. The developments all focus on the prevention of hospitalization, and severe cases after being infected, thus can be used for treating non-hospitalized patients. Pfizer CEO Albert Bourla said in this April, hoping that the use of PF-07321332 can be approved by the end of 2021, with the opportunity to become the first home-cure pill; as for Roche’s AT-527, the first participant was recruited in this April and other 1,386 participants are expected to be recruited by the end of August. It’s also worth mentioning that although the clinical trials of AT-527 still focus on patients with Covid-19, Atea believes that the results of healthy participants show that active metabolite of AT-527 can achieve enough concentration in lining fluid in the lungs, where SARS-CoV-2 replicates. Therefore, it has the potential to be used in disease prevention after exposure to virus, and the experiment in the future is indeed worth attention.
Molnupiravir, the treatment for non-hospitalized patients, mild and moderate cases in the early stage, was co-developed by Merck Sharp & Dohme and Ridgeback.Molnupiravir is a ribonucleoside analog, whose mechanism is to embed genes during the replication of Covid virus to cause mutation, and stop the replication. The U.S. government has agreed to an procurement deal in this June, and it shall come into effect upon Molnupiravir receiving EUA.
Proxalutamide, an androgen receptor antagonist used in the treatment of cancer by Kintor Pharmaceutical in China, was found to decrease the expression of ACE2 and TMPRSS2, further inhibit the entry of the virus into host cells, and stop viral replication. The results of Phase 3 clinical trials in 588 people in Brazil alone show the reduction of mortality rate by 92% in severe cases. More large-scale clinical trials are underway in various centers and countries globally.
The last one is Fujifilm Holdings Corporation’s Favipiravir developed in 1988 for flu, whose patent was expired in many countries. Counrtries around the globe like India, Russia, and China have all launched Phase 3 clinical trials, with a hope to take the lead in the market.
Table 2. Covid-19 Oral Drugs in Late-phase Clinical Trials
Source:Clinicaltrials.gov, official websites in different companies, global news agencies, complied by IMBI & RBMP
An Overview of R&D Progress of Covid-19 New Drugs in Taiwan
■ 9 enterprises in Taiwan has invested in the development of new drugs, and aimed at different markets with diversified drugs development
According to open data from domestic enterprises, those have announced the launch of development in new covid drugs include, PharmaEssentia, Senhwa Biosciences Inc., Foresee Pharmaceuticals Co., Ltd., Golden Biotechnology Corporation, SyneuRx, Oneness Biotech, Advagene Biopharma Co., Ltd., Taiwan Liposome Company, TaiMed Biologics. Immunomodulation and the inhibition of virus are the mainstream mechanisms, with the focus on treatment of mild, severe cases, and respiratory distress syndrome in severe cases. 4 of the drugs are oral dosage forms, which are the most, followed by 3 nasal dosage forms and 2 injection dosage forms. The development of various drugs is to enter different target markets.
Source: Clinicaltrials.gov, Center fo Drug Evaluation, official websites in different companies, global news agencies, compiled by IBMI & RBMP

