

It is hoped that the coronavirus pandemic will soon be curbed in Taiwan with the government’s prompt responses and actions, and cooperation from citizens. Stopping community transmission is of top priority, yet challenges follow simultaneously- such as soaring demands for rapid testing and shortage of PPEs. Three strategies indicated below are aimed at alleviating the pressure of healthcare workers and the level of public panic.
1. Roll out with accurate, rapid and high-throughput RT-PCR tests
The imperative would be for Taiwan to curb SARS-CoV-2 transmission and increase provision of mobile, massive RT-PCR tests. Tests developed with antigen and antibody have been proved with test results suggesting false negative or false positive, which has to be justified eventually with PCR. Now with AI, the virus can even be detected in five minutes from a test taker’s saliva.
Taiwan has technologies allowing rapid RT-PCR testing and capacities for high throughput screening that can be offered by a couple of companies with EUA issuance. The nation should call on a team in place offering mobile, massive RT-PCR tests- with the Ct value generated and shown in a test result- ideally within an hour. In epicentres and national borders, the roll-out of RT-PCR test will ring fence further risk of COVID transmission from and to Taiwan.
2. Coordinate resources much needed by the healthcare system
Industries in Taiwan can help the healthcare system get through hard times by leveraging resources they have. A timely announcement on which supplies of scarcity and of which will be running short, judging by the severity of the virus outbreak, is vital for the industry sector to make informed decisions on ramping up productivity. A quick response gathered from healthcare providers is as follows.
3. Publicly available updates on COVID-related R&D and product development status
The outbreak of Covid-19 since last year has facilitated development of a broad range of products and solutions, resulting in an overall increase in R&D investment, wider medical coverage for those successful ones brought to market and later leading to the public’s panic buying. In addition to the list maintained regularly by TFDA of medical devices granted a EUA approval, it is suggested to provide consolidated information on the current status of COVID-related drugs and vaccines for inquiry, which would be reducing ‘information divide’.
Different Types of COVID-19 Tests
Note: Team Taiwan should aim for developing the test between rapid molecular and RT-PCR standards to deliver objectives of accuracy, speed and ‘high throughput’.
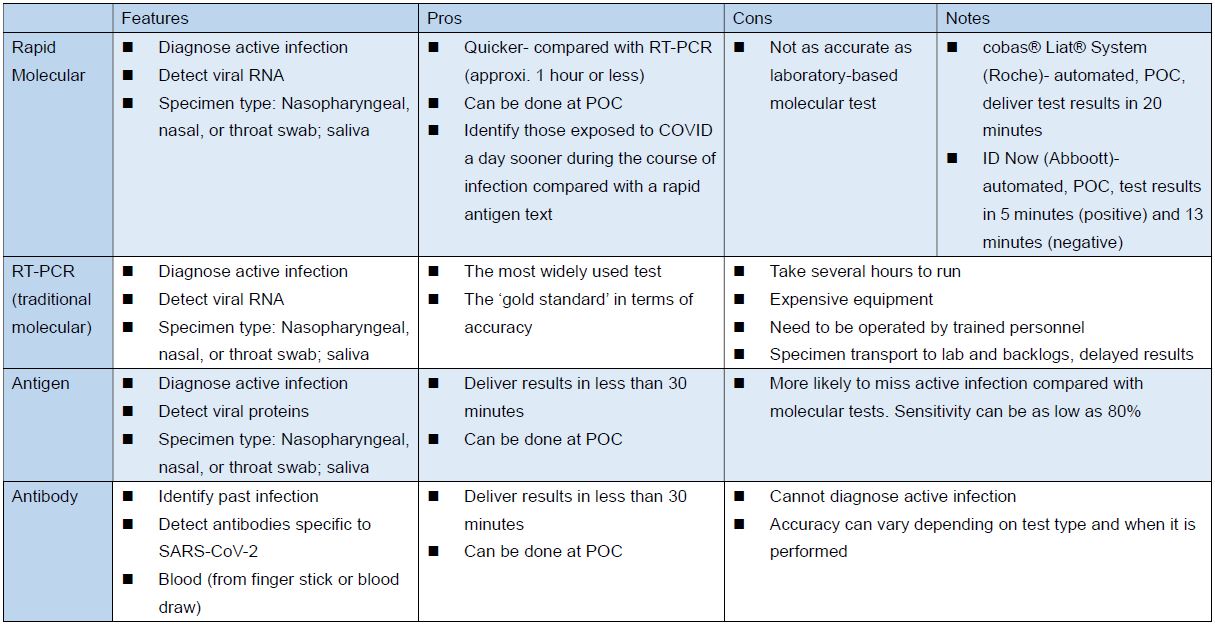
Source: FDA, the Pew Charitable Trusts: COVID-19 Tests Highlight Need for Strengthened FDA Oversight and Diagnostics Legislation; the table above compiled and edited by IBMI

